ResMed ApneaLink Diagnostic Device
€ 2,900.00 SKU: 22354
The ResMed ApneaLink Air is a leading Type III home sleep testing (HST) device, engineered to streamline your OSA diagnostic process. It reliably records up to five channels—effort, flow, pulse, SpO2, and snoring—delivering the comprehensive and accurate data needed for confident clinical decision-making. With its proven ease-of-use and high study success rate, the ApneaLink Air is the ideal, cost-effective solution for efficient sleep apnea screening in any professional setting.
Contact us for a consultation:
Description
ResMed ApneaLink Air: Professional Sleep Apnea Screening

Take the first and most important step towards better sleep with the ResMed ApneaLink Air, a professional home sleep testing device. Trusted by healthcare professionals worldwide, this compact and user-friendly system provides an accurate and reliable screening for Obstructive Sleep Apnea (OSA) from the comfort and privacy of your own bed. No more long waiting lists for sleep labs—the ApneaLink Air empowers you to gather the data needed for a diagnosis, quickly and efficiently.
This device is designed for simplicity, making it incredibly easy for you to perform a professional-level diagnostic test without any prior experience. Simply follow the straightforward instructions, wear the device overnight, and the ApneaLink Air will record key respiratory metrics needed to identify sleep-disordered breathing.
Why Choose Home Sleep Testing with ApneaLink Air?
Sleep apnea is a serious condition that often goes undiagnosed. The ApneaLink Air offers a convenient and cost-effective alternative to in-lab polysomnography, serving as a robust screening tool that delivers clear, actionable results. It’s the perfect solution for anyone who experiences common symptoms like loud snoring, daytime sleepiness, morning headaches, or witnessed breathing pauses during sleep.
Comprehensive and Accurate Data Recording

Despite its small size, the ApneaLink Air is a powerful diagnostic tool that records up to five essential channels of information:
- Respiratory Effort: Measures the expansion and contraction of your chest to determine breathing effort.
- Nasal Flow: A nasal cannula precisely records airflow and detects limitations or stoppages.
- Blood Oxygen Saturation (SpO2): The EasySense finger probe continuously monitors your blood oxygen levels, a key indicator of respiratory events.
- Pulse: Tracks your heart rate throughout the night, which can fluctuate during apnea episodes.
- Snoring: The device differentiates between snoring and normal breathing sounds.
This comprehensive data allows a sleep professional to accurately calculate your Apnea-Hypopnea Index (AHI) and assess the severity of your condition, providing the necessary information to recommend the best course of therapy.
Unmatched Simplicity and User-Friendly Design
The ApneaLink Air was designed with the user in mind. The setup is intuitive, with clearly labeled indicators and a simple one-button start/stop function. The device’s Test Complete light provides instant feedback, confirming that enough data (at least 6 hours) has been recorded for a successful study, giving you peace of mind that the test was performed correctly.
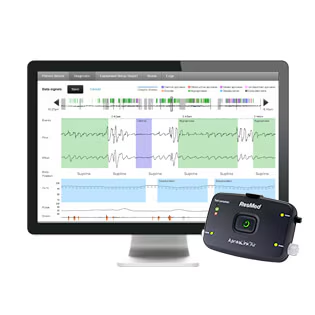
Key Features of the ResMed ApneaLink Air:
- Clinically Proven and Reliable: Used by sleep labs and healthcare providers around the world for accurate OSA screening.
- Easy to Use: Simple setup with intuitive indicators and one-touch operation.
- Comfortable and Compact: Lightweight and non-intrusive design ensures a more natural night’s sleep in your own bed.
- Comprehensive Data Capture: Records up to five channels for a detailed and accurate analysis.
- Instant Test Confirmation: “Test Complete” indicator confirms a successful recording.
- Convenient and Cost-Effective: A practical first step in diagnosing sleep apnea without the complexity of an in-lab study.
In the box
- ApneaLink Air recorder Device
- Carry Bag
- XPOD LP Oximeter and Pulse measurement
- 3 x Pulse Oximeter Sensors (single use)
- 3 x ApneaLink Nasal Cannula (single use)
- XPOD LP Clip
- EasySense Respiratory Effort Sensor
- 1 x Reusable belt
- USB download cable
- ApneaLink Application Software
This KIT comes with single-use Sensors for oximetry and pulse measurement.
Technical specifications
| General Specifications | |
|---|---|
| Device Classification | Type III Home Sleep Testing (HST) Device (according to AASM standards) |
| Primary Use | Screening for Obstructive Sleep Apnea (OSA) in adult patients |
| Regulatory Compliance | Meets IEC 60601-1 and specific regional medical device standards |
| Data Recording Channels & Sensors | |
| Nasal Airflow | Recorded via standard nasal cannula (pressure transducer) |
| Snoring | Detected via the nasal airflow signal |
| Respiratory Effort | Via a reusable respiratory effort inductance plethysmography (RIP) belt |
| Blood Oxygen Saturation (SpO2) | Via the EasySense™ finger pulse oximeter |
| Pulse Rate | Derived from the oximetry signal |
| Physical Specifications | |
| Dimensions (L x W x H) | 111 mm x 61 mm x 52 mm |
| Weight | 66 g (device only, without batteries) |
| Power Source | 2 x AAA alkaline batteries (1.5V) |
| Data Management & Software | |
| Internal Memory Capacity | Sufficient for up to 48 hours of recording |
| Recording Time | Up to 12 hours on a single study |
| Data Transfer | Direct USB connection to a Windows PC |
| Software Compatibility | ResMed AirView™ Diagnostic Software for analysis and reporting |
| User Feedback | “Test Complete” indicator light to confirm sufficient data recording (≥ 6 hours) |
FAQ
1. What is the ResMed ApneaLink Air?
The ApneaLink Air is a Type III Home Sleep Testing (HST) device according to AASM guidelines. It is designed as a robust and efficient screening tool for healthcare professionals to accurately assess adult patients for Obstructive Sleep Apnea (OSA) in the comfort of their own home.
2. Can the ApneaLink Air diagnose Central Sleep Apnea (CSA)?
No. As a Type III device, the ApneaLink Air records respiratory effort via an RIP belt but does not have EEG channels to determine sleep stages or arousals. Therefore, it is primarily designed to screen for obstructive events and cannot be used to definitively diagnose or differentiate Central Sleep Apnea. A full in-lab Polysomnography (PSG) is required for that purpose.
3. What specific parameters does the ApneaLink Air record?
The device records up to five channels critical for OSA assessment:
- Nasal Airflow (via pressure transducer)
- Respiratory Effort (via RIP belt)
- Blood Oxygen Saturation (SpO2)
- Pulse Rate (derived from oximetry)
- Snoring (derived from the airflow signal)
4. How is the data from a study analyzed and reported?
The recorded data is downloaded from the device via USB to a PC running ResMed’s AirView™ Diagnostic Software. The software provides both automated analysis with standard AASM scoring criteria and allows for manual review and editing by a qualified sleep physician. It generates a comprehensive report that includes the Apnea-Hypopnea Index (AHI), Oxygen Desaturation Index (ODI), and other key metrics.
5. What is the “Test Complete” indicator and what is its purpose?
The “Test Complete” indicator is a feature that provides immediate visual feedback on the device itself. A green light confirms that a sufficient duration of data (a minimum of 6 hours) has been successfully recorded. This significantly reduces the rate of failed studies due to patient error, improving workflow efficiency.
6. How does the ApneaLink Air compare to a full in-lab PSG?
The ApneaLink Air is a screening tool, while PSG is the gold standard for diagnosis. The ApneaLink Air provides essential cardiorespiratory data, making it ideal for patients with a high pre-test probability of moderate-to-severe OSA and no major comorbidities. PSG is more comprehensive, recording EEG, EOG, EMG, and other channels, making it necessary for diagnosing complex cases, comorbid sleep disorders, or CSA.
7. Are the sensors and belts reusable?
Yes, most components are designed for multi-patient use. The ApneaLink Air recording device, the reusable effort belt, and the EasySense oxygen probe are all durable and can be cleaned and disinfected according to the user manual’s instructions. The nasal cannula, however, is a single-use disposable item for hygienic purposes.
8. Is special training required for the patient to use the device?
No, the device is designed for self-setup by the patient with minimal instruction. The simple connection points, color-coded components, and one-button operation ensure a high rate of successful first-time use, making it ideal for an efficient HST program.
9. What are the system requirements for the software?
The ResMed AirView™ Diagnostic Software is a cloud-based platform accessible via a web browser on a standard Windows PC. A stable internet connection is required for data upload and analysis. This allows for flexible and remote scoring from any location.
10. Who is the ideal patient candidate for an ApneaLink Air study?
The ideal candidate is an adult patient with a high pre-test probability of moderate to severe Obstructive Sleep Apnea. This includes patients with classic symptoms like loud snoring, witnessed apneas, and significant daytime sleepiness, who do not have major cardiorespiratory comorbidities, neuromuscular diseases, or a suspicion of other sleep disorders like CSA or narcolepsy.















Reviews
There are no reviews yet.